Here, I will post brief answers to the questions arising from students during the Origins of Elements lecture series, part of the ENVIRONMENTAL CHEMISTRY 2 (CHEM08020) module at the School of Chemistry, the University of Edinburgh.
The topic of Nucleosynthesis has been first added to the program in the 2020/21, and therefore, as the lectures continue through the years, I aim to grow and improve this area. For feedback and discussions, please use the comment section at the bottom of the page.
Radioactive Decay Simulation
Click on the Play button to run the code, and on the pencil to edit.
Electron Capture: what happens in the nucleus and where does mass changes come from
While, as chemists, we typically think of atoms being made from neutrons and protons in the nucleus and electrons buzzing around, this view is a little bit simplistic. Let’s have a little zoom into these particles…

Neutrons and protons are composite particles made from quarks. Quarks are elementary particles (i.e. they are not made of other particles) and come in six flavours: up, down, strange, charm, bottom and, obviously, top. They have intrinsic properties, including charge, mass and a spin, see infographic on the right and explore particles interactively here. Then there are, of course, antiquarks. Those are the same but opposite – they are denoted with a bar over the letter of their flavour, so u for ‘up quark’ and ū for ‘up antiquark’. Protons and neutrons are a combination of three quarks: the proton contains two quarks up and one down (“uud”) and the neutron two downs and one up (“ddu”) [can you see where proton’s and neutron’s mass and charge come from?]. To combine, quarks are held together by the Strong force.
In general, we call hadrons particles generated by a combinations of quarks. A hadron made of an odd number of quarks (normally 3) is also called a baryon [remember the Baryon epoch?], while one made of an even number of quarks (typically quark and antiquark) is also called a meson. Just to make things more fun, there are also exotic hadrons, which have been discovered recently (search for ‘Large Hadron Collider beauty’ experiment at CERN).
Electrons are also particles but, unlike neutrons and protons, they are elementary. Electrons are part of a class of particles called leptons. Leptons can be either charged (such as an electron) or neutral (neutrino) and they do not interact via the strong force. In total there are six lepton flavours.
As you can also notice from the schema on the right, there are other particles that carry force – bosons. The Strong force, that holds quarks together, is mediated by gluons; the Electromagnetic force – by photons, and the Weak force is mediated by bosons. W+ and W− bosons (named after Weak interaction) carry a charge and mediate neutrino absorption and emission. The Z0 boson (for Zero charge) mediates the transfer of momentum, spin and energy during neutrino scattering, and is not involved in any nuclear changes. Finally, we should not forget the famous Higgs boson, which is massive and so decays very rapidly. The interaction with the Higgs field is what gives particles their mass – read more here on the CERN page.
Now, since we know what makes up the atom, we can think about the phenomenon of electron capture in a bit more detail. If we have a proton-rich nucleus (in a neutral atom), the nucleus can absorb a nearby electron. The space left empty by this electron is filled by another electron, hopping down from an upper shell. This hop leads to the emission of energy. This energy is either emitted as an X-ray photon or is transferred to the outer electron that is then ejected (Auger effect). Meanwhile, the electron that was captured by the nucleus interacts with a proton, turning it into a neutron and leading to the emission of a neutrino. The proton becomes a neutron because the electron interacts with one of its two up quarks via a W boson. This turns the up quark into a down quark, and leads to the emission of a neutrino.
TOP OF PAGEWhat beryllium-8 can tell us about the UNIVERSE?
In 1939, Bethe was studying the abundance (=stability) of isotopes, and noticed that there is a gap at 5 and 8 (paper here). There is no way to combine just two nuclei and get past this ‘mass gap’. Let’s look at He-burning, which fuels red giants. It starts with a triple-alpha process,a multi-step reaction first described by Salpeter in 1952 (paper here). The fusion of two alpha particles forms Beryllium-8.
4He + 4He ↔ 8Be Q=-0.092 MeV
The nucleus of Beryllium-8 is unstable, and so will decay within a half-life of 8×10-17s. Nevertheless, some small amount will remain and build-up, allowing for the second part of the reaction:
8Be + 4He ↔ 12C Q=7.367 MeV
Now, we have another problem: the rate of conversion into carbon-12 would be too slow to explain the observed galactic abundances. In 1953 Hoyle predicted that carbon-12 should have an excited state with ~ 7.7 MeV energy (paper here) which was later experimentally identified. This nuclear resonance of carbon-12 significantly increases the chances of He combining with Be and it is called Hoyle resonance. See the figure below for the summary:
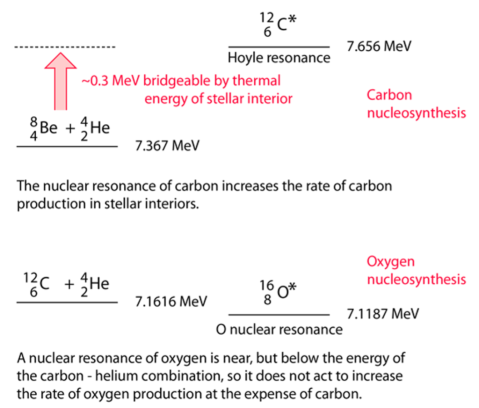
As you can see, the triple-alpha reaction allows to jump the 6-11 nuclides, that are thus not formed during stellar nucleosynthesis.
TOP OF PAGEWhere do Lithium, Boron and Beryllium come from?
Previously we have seen that Beryllium is formed as part of the process of He-burning. However, Beryllium, like Lithium and Boron, has a weakly bound nucleus, that is generally destroyed in stars. To produce these atoms, one would need to collide particles with a lot of energy in an environment where no further reactions can continue, i.e. not in a dense stellar interior. It was calculated that the particles would need to be accelerated to 10%+ the speed of light. So, here is a new question – where do Li, B and Be come from?
In 1970 Reeves, Fowler and Hoyle demonstrated that cosmic rays had the needed energy to create these projectile particles (paper here). This is also Discussed in the book “The Grand Design” by Hawking and Mlodinow, Ch 7:
TOP OF PAGEThis process of carbon creation is called the triple alpha process because “alpha particle” is another name for the nucleus of the isotope of helium involved, and because the process requires that three of them (eventually) fuse together. The usual physics predicts that the rate of carbon production via the triple alpha process ought to be quite small. Noting this, in 1952 Hoyle predicted that the sum of the energies of a beryllium nucleus and a helium nucleus must be almost exactly the energy of a certain quantum state of the isotope of carbon formed, a situation called a resonance, which greatly increases the rate of a nuclear reaction. At the time, no such energy level was known, but based on Hoyle’s suggestion, William Fowler at Caltech sought and found it, providing important support for Hoyle’s views on how complex nuclei were created.
“The Grand Design” by Hawking and Mlodinow, Ch 7
Hoyle wrote, “I do not believe that any scientist who examined the evidence would fail to draw the inference that the laws of nuclear physics have been deliberately designed with regard to the consequences they produce inside the stars.” At the time no one knew enough nuclear physics to understand the magnitude of the serendipity that resulted in these exact physical laws. But in investigating the validity of the strong anthropic principle, in recent years physicists began asking themselves what the universe would have been like if the laws of nature were different. Today we can create computer models that tell us how the rate of the triple alpha reaction depends upon the strength of the fundamental forces of nature. Such calculations show that a change of as little as 0.5 percent in the strength of the strong nuclear force, or 4 percent in the electric force, would destroy either nearly all carbon or all oxygen in every star, and hence the possibility of life as we know it. Change those rules of our universe just a bit, and the conditions for our existence disappear!
